Brand Name
Orladeyo
Generic Name
Berotralstat
View Brand Information FDA approval date: December 04, 2020
Classification: Plasma Kallikrein Inhibitor
Form: Capsule
What is Orladeyo (Berotralstat)?
ORLADEYO ® is indicated for prophylaxis to prevent attacks of hereditary angioedema in adults and pediatric patients 12 years of age and older. ORLADEYO is a plasma kallikrein inhibitor indicated for prophylaxis to prevent attacks of hereditary angioedema in adults and pediatric patients 12 years and older. Limitations of Use : ORLADEYO should not be used for treatment of acute HAE attacks. Limitations of Use : The safety and effectiveness of ORLADEYO for the treatment of acute HAE attacks have not been established. ORLADEYO should not be used for treatment of acute HAE attacks. Additional doses or doses of ORLADEYO higher than 150 mg once daily are not recommended due to the potential for QT prolongation [see Warnings and Precautions.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Orladeyo (Berotralstat hydrochloride)
1INDICATIONS AND USAGE
ORLADEYO
2CONTRAINDICATIONS
None.
3ADVERSE REACTIONS
The following clinically significant adverse reaction is described elsewhere in the labeling:
- QTc Interval Prolongation
3.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
3.2Postmarketing Experience
The following adverse reactions have been identified during postapproval use of ORLADEYO. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal Disorders: nausea
4DESCRIPTION
ORLADEYO (berotralstat) is a plasma kallikrein inhibitor. Berotralstat is presented as the dihydrochloride salt with the chemical name 1-[3-(aminomethyl)phenyl]-
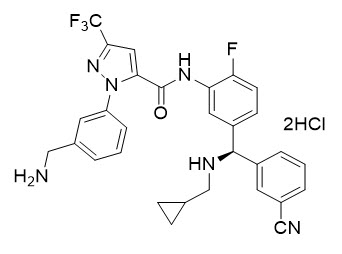
Berotralstat dihydrochloride is a white to off-white powder that is soluble in water at pH ≤4. The molecular formula is C
ORLADEYO (berotralstat) capsules contain 150 mg of berotralstat (equivalent to 169.4 mg berotralstat dihydrochloride) or 110 mg of berotralstat (equivalent to 124.3 mg berotralstat dihydrochloride) in hard gelatin capsules for oral administration. Each capsule contains the active ingredient berotralstat dihydrochloride and the inactive ingredients colloidal silicon dioxide, crospovidone, magnesium stearate, and pregelatinized starch.
ORLADEYO (berotralstat) oral pellets are white to off-white film-coated pellets for oral administration and enclosed in a unit-dose packet containing berotralstat 72 mg (equivalent to 81.3 mg berotralstat dihydrochloride), 96 mg (equivalent to 108.4 mg berotralstat dihydrochloride), 108 mg (equivalent to 122.0 mg berotralstat dihydrochloride), or 132 mg (equivalent to 149.1 mg of berotralstat dihydrochloride). Each unit-dose packet contains the active ingredient berotralstat dihydrochloride and the inactive ingredients colloidal silicon dioxide, crospovidone, magnesium stearate, and pregelatinized starch. The oral pellets film coating contains butylated methacrylate copolymer, silicon dioxide, sodium lauryl sulphate, stearic acid, talc, and titanium dioxide.
5CLINICAL STUDIES
The efficacy of ORLADEYO for the prevention of angioedema attacks in adult and pediatric patients 12 years of age and older with Type I or II HAE was demonstrated in Part 1 of a multicenter, randomized, double-blind, placebo-controlled, parallel-group trial (Trial 1 [NCT3485911]).
The trial included 120 adults and pediatric patients 12 to <18 years of age who experienced at least two investigator-confirmed attacks within the first 8 weeks of the run-in period and took at least one dose of study treatment. Patients were randomized into 1 of 3 parallel treatment arms, stratified by baseline attack rate, in a 1:1:1 ratio (ORLADEYO 110 mg, ORLADEYO 150 mg, or placebo by oral administration once daily, with food) for the 24-week treatment period (Part 1).
Patients discontinued other prophylactic HAE medications prior to entering the trial; however, all patients were allowed to use rescue medications for treatment of breakthrough HAE attacks.
A history of laryngeal angioedema attacks was reported in 74% of patients and 75% reported prior use of long-term prophylaxis. The median attack rate during the prospective run-in period (baseline attack rate) was 2.9/month. Seventy percent of patients enrolled had a baseline attack rate of ≥2 attacks/month.
ORLADEYO 150 mg and 110 mg produced statistically significant reductions in the rate of HAE attacks compared to placebo for the primary endpoint in the Intent-to-Treat (ITT) population as shown in Table 3. The percent reductions in HAE attack rate were greater with ORLADEYO 150 mg and 110 mg relative to placebo, regardless of attack rate during the run-in period.
Reductions in attack rates were observed in the first month of treatment with ORLADEYO 150 mg and 110 mg and were sustained through 24 weeks as shown in Figure 2.
Figure 2. Mean (+/- SEM) HAE Attack Rate/Month Through 24 Weeks (Trial 1) - ITT Population (Adult and Pediatric Patients 12 Years of Age and Older)
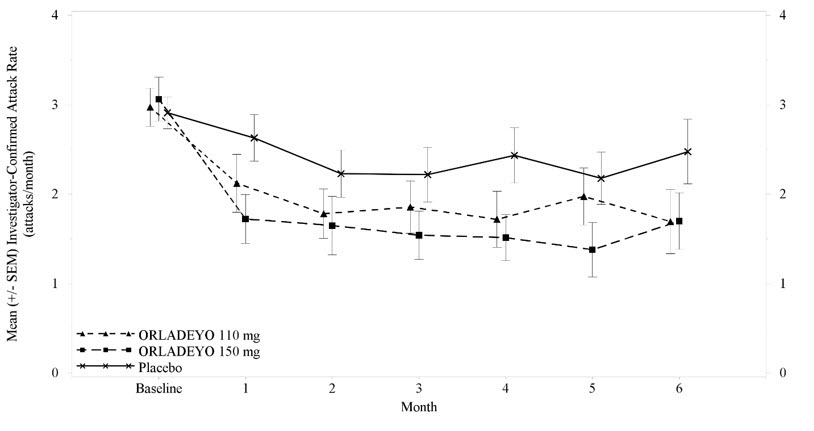
Pre-defined exploratory endpoints included the proportion of responders to ORLADEYO, defined as at least a 50% relative reduction in HAE attacks during treatment compared with the baseline attack rate; 58% of patients who received ORLADEYO 150 mg and 51% of patients who received ORLADEYO 110 mg had a ≥50% reduction in their HAE attack rates compared to baseline versus 25% of placebo patients. In post hoc analyses, 50% and 23% of patients who received ORLADEYO 150 mg, and 27% and 10% of patients who received ORLADEYO 110 mg, had a ≥70% or ≥90% reduction in their HAE attack rates compared to baseline versus 15% and 8% of placebo-treated patients, respectively. The rate of HAE attacks rated as moderate or severe was reduced by 40% and 10% in patients who received ORLADEYO 150 mg and 110 mg, respectively, versus placebo-treated patients.
6HOW SUPPLIED/STORAGE AND HANDLING
ORLADEYO (berotralstat) capsules and oral pellets are supplied as follows in Table 4:
7PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Inform patients of the risks and benefits of ORLADEYO before prescribing or administering to the patient.
8PRINCIPAL DISPLAY PANEL - 150 mg Capsule Blister Card Shellpack Carton
NDC 72769-101-01
orladeyo
FOR ORAL USE ONLY
28 capsules
bio cryst

9PRINCIPAL DISPLAY PANEL - 110 mg Capsule Blister Card Shellpack Carton
NDC 72769-102-01
orladeyo
FOR ORAL USE ONLY
28 capsules
bio cryst

10PRINCIPAL DISPLAY PANEL - 72 mg Pellet Packet Wallet Carton
NDC 72769-111-02
orladeyo
72 mg per packet
28 Packets
Lift here to open

11PRINCIPAL DISPLAY PANEL - 96 mg Pellet Packet Wallet Carton
72769-112-02
orladeyo
96 mg per packet
28 Packets
Lift here to open

12PRINCIPAL DISPLAY PANEL - 108 mg Pellet Packet Wallet Carton
72769-113-02
orladeyo
108 mg per packet
28 Packets
Lift here to open

13PRINCIPAL DISPLAY PANEL - 132 mg Pellet Packet Wallet Carton
72769-114-02
orladeyo
132 mg per packet
28 Packets
Lift here to open
